
Niger J Paed 2016; 43 (1):30 – 33
ORIGINAL
Ocheke OI
The febrile child: how frequent
John CC,
Ogbe P,
should we investigate for urinary
Donli A,
tract infection
Oguche S
DOI:http://dx.doi.org/10.4314/njp.v43i1.6
Accepted: 3rd August 2015
Abstract :
Background: Febrile
where necessary: blood film for
illness in children remains the
malarial parasite identification and
Ocheke OI (
)
most common cause of emer-
count, cerebrospinal fluid (CSF)
John CC, Ogbe P, Donli A, Oguche S
gency room visit. In many tropical
analysis and chest X-ray.
Department of Paediatrics,
Jos
University Teaching Hospital
countries where malaria is en-
Results: Of
the 303
children 180
P.
M. B 2076, Jos, Nigeria
demic, children presenting with
(59.4%) were males and 123 were
Email: ieocheke@yahoo.com
fever are treated for malaria pre-
females (40.6%). The mean age
sumptuously. Current evidence
was 21.7±14.0months, 54.5% were
suggests however that malarial
less than 24months.
parasitaemia in febrile children is
ARI accounted for 44.6% (mainly
declining and the prevalence of
tonsillitis, 61%, pneumonia, 27%
other causes of fever apparently
and otitis media, 12%), while ma-
on
the increase. Therefore, high-
laria and UTI were observed in
lighting such causes of fever as
38.3% and 4.6% respectively.
urinary tract infection (UTI) is
Five (35.7%) patients with UTI
indispensable. This is much so as
were males while 9 (64.3%) were
UTI not only is common in
females. Their combined mean age
younger children and often ne-
was 25.4±18.6months, 57% of
glected but also associated with
these children were less than 24
long term complications
months old. In 3(21.4%), UTI co-
Methods:
Children
aged
6-
existed with malaria.
59months with fever of less than
Conclusions: Acute
respiratory
2weeks were consecutively re-
infection, malaria and UTI are the
cruited. Each child had both clini-
three leading causes of fever in
cal evaluation and preliminary
children under 5 years.
laboratory assessment such as
dipstick urinalysis. Further micro-
Keywords: Fever,
Children, Acute
biological
and
radiological
respiratory infection, Malaria,
evaluations
were
performed
UTI.
Introduction
malaria parasitaemia accounted for only 35 % of 5,217
children presenting with fever.
8
Febrile illness represents the most common cause of
The pre-treatment identification of malaria parasites in a
hospital visits and admission in children globally.
1,2
The
suspected case is beneficial in many ways much as only
underlying causes are predominantly infectious and UTI
individuals with the infection are treated. A new prob-
is
increasingly recognised as an important cause. Ap-
3
lem as a result of this step however is, what appropriate
proximately two decades ago, it was noted that 30% of
treatment would those who test negative for
Plasmo-
outpatient and 50% of inpatient children under the age
dium falciparum parasites
receive, particularly at
the
of
five years living in Sub-Saharan Africa had malaria.
4
first and secondary-level health facilities. Available evi-
Without a reliable case definition, children presenting
dence suggests that with the decrease in the use of anti-
with febrile illness in most cases were treated presump-
malarials, there has been a corresponding increase in the
tively for malaria.
1,5-6
Current evidence suggests how-
indiscriminate use of antibiotics in febrile children.
9,10
ever that there is declining malarial transmission in
Many times, the choice of antibiotics and their dosages
many of the malarious countries in Africa. This necessi-
are inappropriate, inadequate and unnecessary. This
tated the World Health Organisation (WHO), point-of-
practice can lead to increase in the development of sev-
care rapid diagnostic test which ensures that all children
eral resistant microbial strains. It could also lead to poor
presenting with fever are tested for malaria before em-
care and missed diagnosis of bacterial infections that
barking on antimalarial treatment. In Nigeria a recent
7
may result in long term complications particularly in
younger children.
11
multicentre study conducted in 2009/2010 showed that

31
It
has been shown that urinary tract infection (UTI) is
Laboratory assessment
common in children presenting with febrile illness par-
ticularly in younger age groups, and could be associated
A
dipstick urinalysis was done on a portion of urine
12,13,14
with long-term complications.
These complications
specimen (catheter urine and clean catch for children 2
could be prevented by early diagnosis and prompt treat-
years and below, while mid-stream urine for older ones),
ment.
for
each subject who did not have any obvious focus of
infection. This was to identify the presence of nitrite and
Considering these, a systematic approach to case man-
or
leukocyte esterase (LE). Only the urine of children
agement of febrile children, particularly those under-five
whose dipstick urinalysis was positive for both nitrite
years is imperative. Such a measure would include care-
and LE or for either of the two were subjected to further
ful clinical evaluation and laboratory assessment to iden-
microbiological analysis according to National Institute
for Health and Care Excellence (NICE) guidelines.
16
tify the underlying cause of fever in each child. This
study was therefore, undertaken to highlight the underly-
The remaining portion of such urine sample was sent to
ing causes of fever in children six months to 59 months
the JUTH microbiology laboratory for microscopy, cul-
with specific emphasis on UTI identification in our hos-
ture and sensitivity within one hour of collection accord-
pital.
ing to standard protocol. The confirmation of UTI was
made only in children with a single bacterial isolate of
≥10 colony forming units (CFU) per ml of mid-stream
5
17
urine and 10 CFU/ml of catheter urine.
3
Materials and methods
Venous blood was obtained for peripheral smear and
subjected to Giemsa stain for
Plasmodium falciparum
This was prospective, cross-sectional and descriptive
identification and counting. Similarly, all children with
study, carried out in the paediatric emergency and outpa-
features suggestive of acute central nervous system in-
tient units of the Jos University Teaching Hospital, Ni-
fections (fever with seizures, nuchal rigidity or uncon-
geria. Study participants were children aged 6 to 59
sciousness) had lumbar punctures for cerebrospinal fluid
months seen in these units from the first of April to the
(CSF) analysis.
end of October 2012. Ethical approval was obtained
Data obtained were entered into EPI info version
from the Institution's Health Research and Ethics Com-
3.4.3software for analysis. The student‘t’ test was used
mittee. All children who presented with fever (axillary
to
compare group means and the Chi-squared test to
temperature ≥ 37.5 C), that had lasted less than two
o
compare proportions. Fisher exact was used when cells
weeks and whose parental/caregiver consent had been
contained observations less than 5. P value less than
obtained were consecutively recruited. Children with
0.05 was considered significant.
moderate to severe malnutrition according to world
health organization (WHO)
15
reference charts, those
with sickle cell anaemia or any underlying chronic ill-
nesses (renal, cardiac and chronic infection such as tu-
Results
berculosis or human immunosuppressive virus) were
excluded. Similarly, children who had used antibiotics
A
total of 303 children were recruited for the study. One
within one week prior to hospital visit were not in-
hundred and eighty (59.4%) were males while 123
cluded. All the children seen during the study, including
(40.6%) were females. Table 1 show the clinical charac-
those who participated and those who did not were
teristics of the subjects. The combined mean age for
given appropriate medical care following careful evalua-
both males and females was 21.67±14.02 while the me-
tion.
dian age was 17 months. One hundred and ninety nine
(65.7%) were aged less than 24 months.
Clinical assessment
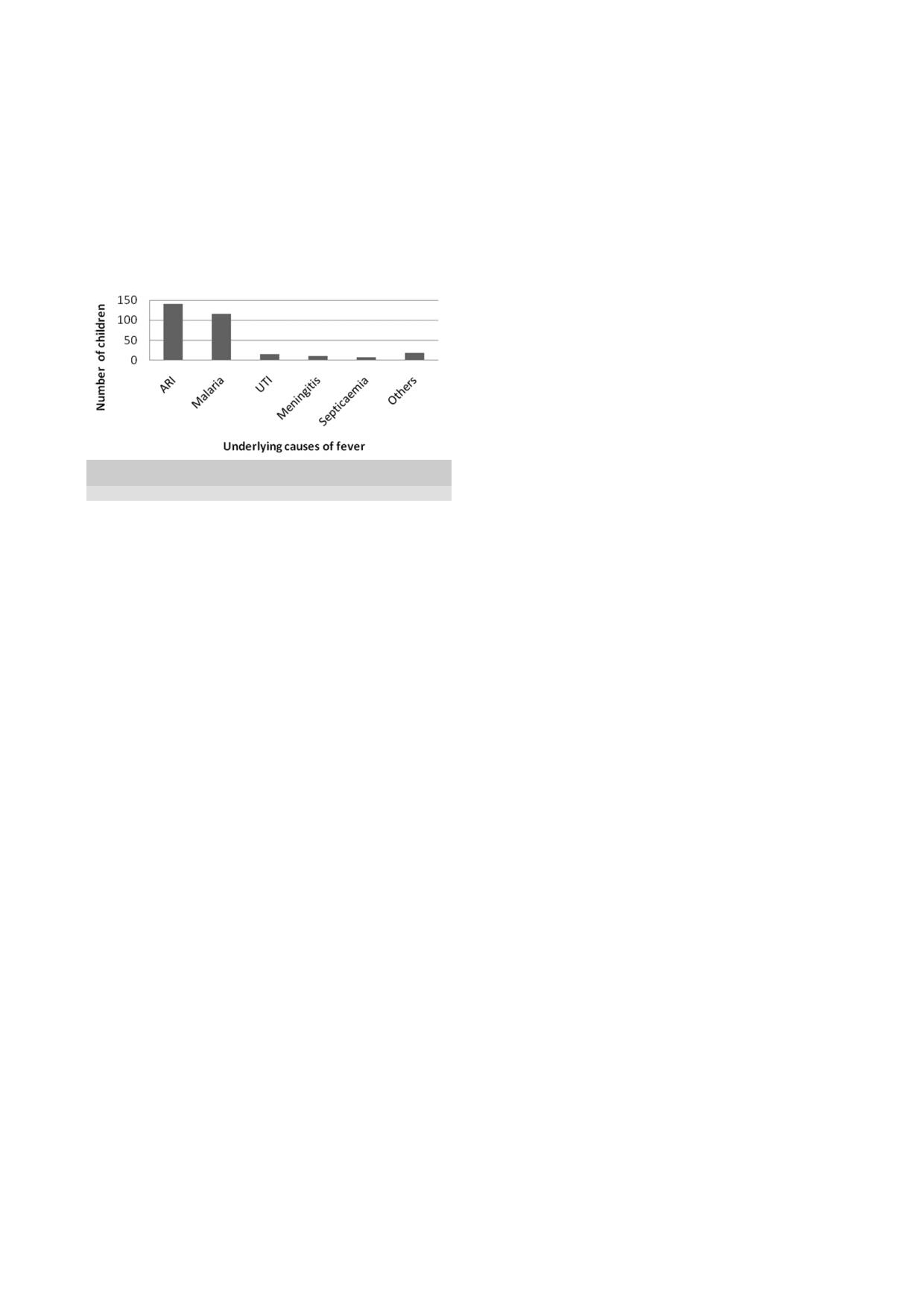
Fig 1 shows the causes of fever in the study population.
The history of illness was obtained from the patient’s
Acute respiratory infection, comprising tonsillitis, pneu-
parent/caregiver. Each child had physical examination to
monia and otitis media in that order, was the leading
identify possible focus of infection or any other feature
cause of fever in the children, constituting 44.6%. Ma-
that could assist in making diagnosis. Those who have
laria was the second common cause of fever followed by
clinical features suggestive of acute lower respiratory
urinary tract infection at 38.3% and 4.6% respectively.
infections had chest radiography. All participating sub-
Of
the children with ARI, 93 (69%) were aged 24
jects had their weight taken using a Seca® standing or
months and below with majority, 54 (58.1%) in this
bassinet weighing scales (infants), while height/length
group aged 12 months and less. Similarly, 86 (62.4%) of
was
measured in recumbent or standing positions based
the
children who had malaria were aged 24 months and
on
the age of the child respectively.
below. There were 14 children with UTI, 5(35.7%) in
The mid upper arm circumference (MUAC), was meas-
males and 9(64.3%) in females. Their mean age was
ured in all the children using an inelastic tape and meas-
25.4 ± 18.6 months. Eight children (57%) were 24
urement recorded to the nearest 0.1cm.
months or less while the remaining 6 (43%) patients
were older.
Three of the children with UTI also had parasitological
evidence of malaria. The commonest organism
32
responsible for UTI was
Escherichia coli in
8(57.1%).
graphical location may also have contributed as the find-
The other organisms included Klebsiella
species in 3
ing from Tanzania showed; the pattern of infection in
(21.4%), Staphylococcus
aureus 2(14.3%) and
Proteus
febrile children was different between the mainland and
species in 1(7.1%). A significantly high proportion
Zanzibar Island.
(86.4%) of the organisms isolated were sensitive to
flouroquinolones, sensitivity to gentamicin was 56.8%,
The prevalence of UTI in our study of 4.6% is much
while to the third generation cephalosporin (ceftriaxone)
lower than two previous and similar studies from other
parts of Nigeria.
22,23
Ibeneme et al
22
was 46.2%.
reported a UTI
prevalence of 11% in febrile children aged 1 to 59
Fig 1: Underlying
diagnosis of
fever in
the children
months in South East Nigeria. Their study included chil-
(Others- cellulitis, chicken pox, measles and mumps)
dren of much younger age than ours. It is known that the
prevalence of UTI is higher in younger infants, particu-
larly in the first few months of life.
12,14
Furthermore,
urine culture for microbiological confirmation of UTI
was done for all their study population without initial
screening for nitrite and LE, while we relied on positive
urine nitrite and/or LE for further urine culture. Conver-
sion of nitrate to nitrite does not occur with all bacteria
and it takes about 4 hours or more for conversion of
urine nitrate to nitrite in the bladder.
24
Therefore, if a
child had not retained urine for such period, it was pos-
Table 1: Characteristics
of the
study subjects
sible the screening will be falsely negative even where
Variable
Total
Male
Female
P
value
UTI was present. In their study from north western Ni-
Study population
303
180
123
-
geria, Wammanda et al
23
reported a UTI prevalence of
Age(months)
21.7 ±14.02
21.1 ±13.9
22.6 ±14.3
0.72
(Mean±SD)
24.3% in 185 febrile children who had symptoms refer-
Axillary Temp ( C)
o
38.2 ±0.78
38.2 ±0.76
38.2 ±0.81
0.73
able to the urogenital system. This figure is equally
(Mean±SD)
much higher than our finding. The higher rate they re-
Duration of fever
5.6±4.3
5.3±3.6
5.4±3.5
0.82
before presentation
ported is likely attributable to the fact that only those
(days) (Mean±SD)
children who had urinary signs and symptoms were re-
cruited. In that study, they also compared the ability of
positive urine nitrite to detect UTI with urine culture and
noted that nitrite was less sensitive but had an excellent
specificity. In other words, there is likelihood for this
23
Discussion
test
not to detect UTI even where infection is present.
From
our study, acute respiratory infection was identi-
fied
as the leading infection in children presenting with
Our
study evaluated children for UTI only if they had no
fever, followed by malaria. Urinary tract infection was
focus of infection and where preliminary screening for
the third common cause of fever in these children. This
nitrite and LE was positive. It has been shown that nei-
general pattern is similar to findings from other studies
ther
absence nor presence of a focus of infection neces-
sarily affects the existence of UTI in children. This may
14
in
Africa and elsewhere in the world.
18,19,20,21
In
a study
of
418 children with fever in Gabon, Bouyou-Akotet et
have contributed to the lower prevalence rate observed
al
reported UTI prevalence of 4.1%, while D’Acre-
18
in
this study.
mont et al , in his review of the causes of fever in 1005
19
The prevalence of UTI in febrile children in our study
Tanzanian (Zanzibar) children 2 months to 10 years re-
may have come in a distant third position, but it empha-
ported a prevalence of 5.9%. These studies also showed
sise a significant point that this condition is to be looked
that acute respiratory infection was the commonest
for in children presenting with fever when there is no
cause of fever in the children as our study demonstrated.
focus of infection. This is more so that managing the
We
found that malaria was the second most common
long term complications associated with renal scarring
cause of fever similar to the report from Gabon,
18
but
from pyelonephritis is much more challenging and diffi-
this contrasts with the observation from Zanzibar
19
cult in our environment.
where malaria was the fourth common cause of fever.
Our study has some limitations: urine culture was car-
This variation may be related to capacities for further
ried out only in children whose dipstick urinalysis was
laboratory investigations, environmental and weather
positive for nitrite and/or LE. There may have been
conditions. For instance, in mainland Tanzania, the com-
some of these children whose dipstick urinalysis was
monest cause of fever among 870 paediatric and adult
negative for nitrite and LE but who may actually have
patients was bacterial zoonoses while malaria was re-
UTI. However, this study has shown that, for children
sponsible in only 1.6%. Similarly, among 1180 hospi-
20
presenting with fever in our environment, acute respira-
talized Cambodian children under 8 years with 1225
tory infection is the commonest condition, followed by
febrile episodes, Chheng et al reported that acute respi-
21
malaria and then UTI, stressing the need to evaluate
ratory infection was the foremost cause of fever fol-
such children with this order in mind.
lowed by different kinds of viral infections. The geo-

33
Acknowledgments
Mrs Carol Okorie for carefully analysing the entire
blood specimen for malaria parasites for the children.
We
are grateful to all the children and their parents that
participated in this study. We also acknowledge
Conflict of interest: None
Funding: None
References
1.
Kallander K, Nsungwa-Sabiiti J,
9.
Roll Back Malaria. World malaria
18.
Duguid JP, Marmaion BP, Swain
RH.
Medical Microbiology. 13
th
Peterson S. Symptom overlaps for
report. 2005. http://www.rollback
malaria and pneumonia-policy
malaria.org/wmr2005.
ed.
Edinburgh: Churchill
Living-
implications for home manage-
10.
O'Meara WP, Bejon P, Mwangi
stone; 1978. p. 327-33
ment strategies. Acta
Trop.
TW,
Okiro EA, Peshu N, Snow
19.
Bouyou-Akotet MK, Mawili-
2004;70:211 – 214.
RW,
Newton CRJC, Marsh K.
Mboumba DP, Kendjo E, Ekoum
2.
Liu
L, Johnson HL, Cousens
Effect of a fall in malaria transmis-
AE,
AbdouRaouf O, Allogho EE
S,Perin J, Scott S, et al. Global,
sion on morbidity and mortality in
et
al. Complicated malaria and
regional, and national causes of
Kilifi, Kenya. Lancet.
2008; 70:
other severe febrile illness in a
child mortality: an updated sys-
1555 – 1562
pediatric ward in Libreville, Ga-
tematic analysis for 2010 with
11.
Guerra CA, Gikandi PW, Tatem
bon. Infect
Dis 2012;
12:216-225.
time trends since 2000. Lancet
AJ,
Noor AM, Smith DL, Hay SI,
20.
D’Acremont V, Kilowoko M,
2012; 379: 2151 – 2161
Snow RW. The limits and intensity
Kyungu E, Philipina S, Sangu W,
3.
Rougemont A, Breslow N, Bren-
of Plasmodium falciparum
trans-
Kahama-Maro J et al. Beyond ma-
ner
E, Moret AL, Dumbo O, Dolo
mission :
implications for
malaria
laria — causes of fever in outpatient
A,
Soula G, Perrin L. Epidemiol-
control and elimination worldwide.
Tanzanian children. N
Engl J
Med
ogical basis for clinical diagnosis
PLoS Med. 2008;70:e38. doi:
2014; 370:809-817.
of
childhood malaria in endemic
10.1371/journal.pmed.0050038.
21.
Wolter N, Cohen C, von Gottberg
zone in West Africa. Lancet
12.
Lin DS, Huang SH, Lin CC, Tung
A.
Causes of fever in outpatient
1991;70:1292 – 1295
YC,
Huang TT, Chiu NC et al.
Tanzanian children. N
Engl J
Med
4.
Akpede G.O. and Skyes R.M.
Urinary tract infection in febrile
2014;370(23):2243-2248.
(1992) Relative contribution of
infants younger than eight weeks
22.
Chheng K, Carter MJ, Emary K,
bacteremia and malaria to acute
of
age. Pediatrics
2000;105:E20
Chanpheaktra N, Moore CE,
fever without localizing signs of
13.
Crain EF, Gershel JC. Urinary
Stoesser N et al. A prospective
infections in under five children.
J
tract infection in febrile infants
study of the causes of febrile ill-
Trop Pediatr38, 295-298.
younger than eight weeks of age.
ness requiring hospitalization in
5.
English M., Reyburn H., Good-
Pediatrics 1990; 86:363-367.
children in Cambodia. PLoS
One
man
C. and Snow R.W. (2009)
14.
O’Brien K, Edwards A, Hood K,
2013;8(4): e60634.
Abandoning presumptive antima-
Butler CC. Prevalence of urinary
23.
Ibeneme CA, Oguonu T, Okafor
larial treat-ment for febrile chil-
tract infection in acutely unwell
HU,
Ikefuna AN, Ozumba UC.
dren aged less than five years- a
children in general practice: a pro-
Urinary tract infection in febrile
case of running before we can
spective study with systematic
under five children in Enugu,
walk? PLoS
Med 6, e1000015.
urine sampling. Br
J Gen
Pract
South Eastern Nigeria. Niger J
Clin
6.
D’Acremont V, Lengeler C, Gen-
2013;
63(607):156-164.
Pract 2014;17:624-628.
ton
B. Reduction in the proportion
15.
World Health Organisation, UNI-
24.
Wammanda RD, Aikhionbare HA,
of
fevers associated with Plasmo-
CEF. WHO growth standards and
Ogala WN. Use of nitrite dipstick
dium falciparum parasitaemia in
the
identification of severe acute
test in the screening for urinary
Africa: a systematic review.
Malar
malnutrition in infants and chil-
tract infection in children. West Afr
J 2010;9:240-249.
dren. A joint statement by the
J Med 2000; 19: 206-208.
7.
Gething PW, Patil AP, Smith DL,
World Health Organisation and the
25.
Jodal U. Urinary tract infection:
Guerra CA, Elyazar RF, Johnston
United Nations Children's Fund.
significance, pathogenesis, clinical
GL
et al. A new world malarial
Geneva: WHO, 2009.
features and diagnosis. In: Pos-
map: Plasmodium falciparum
URL:www.who.int/nutrition/
tlethwaite RJ, editor. Clinical Pae-
endemicity in 2010. Malar
J
publications/severemal nutri-
diatric Nephrology. Oxford: But-
2011; 10: 378-386.
tion/9789241598163/en/index.html
terworth - Heinemann; 1994. pp.
8.
Oguche S, Okafor HU, Watila I,
(accessed 21 May 2012).
151
– 9.
Meremiku M, Agomo P, Ogala W
16.
National Institute for Health and
et
al. Efficacy of Artemisinin-
Care Excellence (NICE). Urinary
Based Combination Treatments of
tract infection in children. 2007.
Uncomplicated Falciparum Ma-
http://guidance.nice.org.uk/CG05.
laria in Under-Five-Year-Old Ni-
gerian Children. Am.
J. Trop.
Med.
Hyg., 91(5), 2014, pp. 925 – 935